My own Simulation Activity.
Simulation Used: Molecules and Light
Standards Met: A.12.7 Re-examine the evidence and reasoning that led to conclusions drawn from investigations, using the science themes
C.12.3 Evaluate* the data collected during an investigation*, critique the data-collection procedures and results, and suggest ways to make any needed improvements
G.12.1 Identify personal interests in science and technology, implications that these interests might have for future education, and decisions to be considered
Things to be completed.
1. Create a data table that the student believes accurately shows how different molecules effect different forms of light. Then peer review another students table and critique the method and organization used.
2. Examine the evidence gained from these data tables. What molecules interfere with infrared light? Visible light? Ultraviolet? Explain.
3.Explain why certain types of light are effected by certain molecules
4. How is this data relevant to previous scientific advancements? (for example microwave ovens)
5. What modern careers could be explored using this simulator?
Hypothesis Question
After looking at other students blogs, many of us developed a hypothesis that stated that cold water would in fact freeze faster, because its core temp is closer to the freezing temp. After completing the assignment and researching on my own I figured out this isn't the case. Known as the Mpemba effect, the faster evaporation of water causes less space to freeze compared to the colder water. I did not think this would be the case at all. I think we all sort of developed our hypothesis just using common sense, like cold water would freeze faster because it's colder. After research I learned this was false.
Friday, January 20, 2017
Assignment 8 Part 1
Directions:
1.
Define
“light photons” and “infrared photons”
Light Photons: a particle
representing a quantum of light or other electromagnetic radiation.
Infrared Photons: A photon whose frequency is in the infra-red spectrum: about 1
to 400 THz.
- How are they
represented in the simulation?
-
With yellow and
red lights
- If you were talking
to a friend about what you observe, how would you explain what is
happening with the energy from the sun and the energy from the Earth?
-
Infrared photons
leave the earth as energy along with sunlight photons coming from the sun.
Greenhouse gases keep these photons in the atmosphere causing rising temps.
2.
In
the winter, weather reporters often day “It will be a very cold night because
there are no clouds.”
a.
Use
the sim to see if you can understand why this could be true.
Clouds
can act as ways for photons to stay in the atmosphere, warming the temp.
b.
Describe
your observations.
The
clouds kept the photons in the atmosphere longer, increasing the temp.
c.
If
you were a weather person, how might you use what you understand about clouds
and the effect on temperature to predict night-time weather for a summer month
like June?
Knowing
the sky will be clear, you can inform people that it will be a cooler summer
night.
- How can you make the
greenhouse gases act similar to clouds?
a.
Explain
what you did.
I
increased the greenhouse gas concentration, creating a smog that acts like
clouds.
b.
Give
the evidence to prove you made them act alike in a few different situations.
I
tested this theory in multiple time periods (1750 and today) and the results
were very similar.
- What do you notice about greenhouse gas effect on
photons that is different from clouds? Give examples from situations that
you made in the sim to support your ideas.
-
Greenhouse gases
caused drastic changes in temperature, while cloud coverage changed the temp by
a maximum 15-20 degrees.
5.
Use the Photon
Absorption tab to identify which of the molecules provided in the sims are Greenhouse
Gases. State microscopic evidence that supports your ideas.
-
Methane, Nitrogen, and CO2 all deterred photons
from getting out of the atmosphere at a microscopic level.
Why do you think the inside of a car feels so
much warmer than its surroundings on sunny days?
- How can you use the sim to test your ideas?
-The
greenhouse gases can be similar to a car. The heat is trapped inside creating a
much warmer temperature than the surrounding area.
- Describe your experiment and state some evidence
that explain the different temperatures on a microscopic level using
photons.
-
CO2 was the
biggest problem as a greenhouse gas. No other molecule caused photons as many
problems getting past than C02
Assignment 7
1.Student directions pH
scale activity 1: Introduction to pH
Directions: Use specific examples to demonstrate each
of the following learning goals.
- Determine
if a solution is acidic or basic using
- pH:
- molecular
representation
- Hydronium/Hydroxide
concentration
A. In the simulation using the device ( I assume it's some sort of pH reader) I was able to measure exactly how acidic or basic a liquid was. The pH scale helped me figure out what liquids were acids and which were bases. If you don't have a device
B. By using water and examining the molecular formation of the acid being tested, you can notice distinct characteristics that tell you how strong an acid or a base is. For example, strong acids break apart in water creating H+ and A- ions.
C. Acidity is determined by the concentration of Hydronium in the given solution. the same goes with bases but with hydroxide concentrates. Both of these measurements can determine if a solution is basic or acidic.
C. Acidity is determined by the concentration of Hydronium in the given solution. the same goes with bases but with hydroxide concentrates. Both of these measurements can determine if a solution is basic or acidic.
- Relate liquid color to pH
The colors many times can give away weather a liquid is acidic or basic. Many times acids are clear liquids while bases are commonly are clouded liquids. For example milk is an easy base to recognize while battery acid is an easy acid to recognize. Using a universal indicator solution will change acids from green to red, while bases will turn purple.
- Predict if dilution and volume will increase, decrease or not change the pH
These both will increase the pH. For example, in the simulator, adding water to an acid made the solution less acidic on the pH scale, which in turn increased the pH.
- Organize a list of liquids in terms of acid or base strength in relative order with supporting evidence.
Acids
1. Battery Acid
2. Soda
3.Chicken Soup
Bases
1.Drain Cleaner
2.Hand Soap
3.Blood
Both of these lists were composed using information retrieved from the simulation.
- Write
the water equilibrium expression. Describe how the water equilibrium
varies with pH.
Kw, is 1.01 × 10-14 at 25 °C. The equation will change with varying H+ ions and Hydroxide ions found in both acids and bases.
Click Questions
1. False. The best way to know if a solution is either basic or acidic is by properly testing it with a pH reading device, litmus paper , or the pH equation.
2.D, Both solutions over 7 pH are bases.
3.C, The liquid consists mostly of H+ Ions
4.C. C had the most H30+ ions
5. B. Had the most H+ ions out of the three
6.A. Water being added decreases acidity, which makes the pH go up.
7. B, More water will decrease the liquids base.
8. ABC. A is the lowest pH (Acid) and the other two than increase the pH as bases.
9. CBA. The higher the H+ ions in a liquid the lower the pH
10. A. The solution wasn't at 7 so something was added to make it more basic.
Questions:
1. Very concentrated
1. Very concentrated
2. Strong Electrolyte
3. Weak electrolyte
4. Lower
5.Decreases
6. Increases
7.Decreases
8. Increases
9.Stays the same
10. Decreases
11. Weak acid = 4.79/ Strong acid = 2.00
12. Strong acids will have a lower pH than weaker acids, so the results make sense.
Thursday, January 19, 2017
Assignment 6
1. 0 Degrees F = 255.372 K
32 Degrees F= 273.15 K
70 Degrees F= 294.261 K
212 Degrees F= 373.15 K
Worksheet drawings/ Answers
4. Kelvin
5. The water molecules start to move away from each other and move more freely
6. 328 Degrees K/ 157 Degrees K
7. Below it the molecules stick close together, while above it they move around freely
9. 809 Degrees K
32 Degrees F= 273.15 K
70 Degrees F= 294.261 K
212 Degrees F= 373.15 K
Worksheet drawings/ Answers
4. Kelvin
5. The water molecules start to move away from each other and move more freely
6. 328 Degrees K/ 157 Degrees K
7. Below it the molecules stick close together, while above it they move around freely
9. 809 Degrees K
10. The water below the boiling point vibrate and
move somewhat quickly around. Above the boiling point the molecules vibrate
violently and move around at fast speeds taking up more space.
13.The molecules tended to stay close together in the solid form, but the neon stayed together and didn’t vibrate and move as much as the water did. In liquid form, the substances acted similar, but the neon molecules would sometimes break away from each other and individually float around the container. The gas form of neon seemed much less hectic and smoother moving than the water was.
14.Yes, I knew that solid’s molecules would stay closer together while water would start to make a flowing motion. The gas that I drew also showed molecules all over the place and showed hectic, fast movement.
3. The water molecules bunch together tightly when the temperature is at 0 Degrees K. The Hydrogen molecules tend to become attracted to the other oxygen molecules and vice versa. This keeps each molecule close together.
4. If this was the case for water, ice cubes would sink to the bottom if put in water. Ice cubes float on top of water, so the molecules become attracted to each other, but not close enough to increase the density.
5. 55 ATM 85 Degrees K
6.
7. A.4.1 When conducting science investigations, ask and answer questions that will help decide the general areas of science being addressed
C.4.6 Communicate the results of their investigations in ways their audiences will understand by using charts, graphs, drawings, written descriptions, and various other means, to display their answers
13.The molecules tended to stay close together in the solid form, but the neon stayed together and didn’t vibrate and move as much as the water did. In liquid form, the substances acted similar, but the neon molecules would sometimes break away from each other and individually float around the container. The gas form of neon seemed much less hectic and smoother moving than the water was.
14.Yes, I knew that solid’s molecules would stay closer together while water would start to make a flowing motion. The gas that I drew also showed molecules all over the place and showed hectic, fast movement.
3. The water molecules bunch together tightly when the temperature is at 0 Degrees K. The Hydrogen molecules tend to become attracted to the other oxygen molecules and vice versa. This keeps each molecule close together.
4. If this was the case for water, ice cubes would sink to the bottom if put in water. Ice cubes float on top of water, so the molecules become attracted to each other, but not close enough to increase the density.
5. 55 ATM 85 Degrees K
6.
7. A.4.1 When conducting science investigations, ask and answer questions that will help decide the general areas of science being addressed
C.4.6 Communicate the results of their investigations in ways their audiences will understand by using charts, graphs, drawings, written descriptions, and various other means, to display their answers
Wednesday, January 18, 2017
Assignment 5
1.
2. Density is mass per unit volume. Density = Mass/Volume
3.
A: Gold, Mass= 65.14, Volume=3.38, Density= 19.3
B: Apple, Mass=.64, Volume= .64, Density= .64
C: Water, Mass=4.08, Volume=4.08, Density= 1
D Water, Mass= 3.10, Volume= 3.10, Density=1
E Diamond, Mass = 3.53, Volume= 1.00, Density= 3.53
2. Density is mass per unit volume. Density = Mass/Volume
3.
A: Gold, Mass= 65.14, Volume=3.38, Density= 19.3
B: Apple, Mass=.64, Volume= .64, Density= .64
C: Water, Mass=4.08, Volume=4.08, Density= 1
D Water, Mass= 3.10, Volume= 3.10, Density=1
E Diamond, Mass = 3.53, Volume= 1.00, Density= 3.53
Sunday, January 15, 2017
Assignment 4
Standard A 4.5: When studying a science-related problem, decide what changes over time are occurring or have occurred
-In high school biology, we performed a series of experiments on pea plants to see if water was the best way to get plants to grow. We would have to check our plant's growth progress every other day to see if changes have occurred. This allowed us to examine how the plants changed over time and how it was occurring.
B.4.2 Acquire information about people who have contributed to the development of major ideas in the sciences and learn about the cultures in which these people lived and worked.
-Throughout my k-12 science classes, we would learn about the scientists who discovered and developed the experiments/ theories that we were currently learning about. We would use various resources to help study these important scientists.
C.4.2 Use the science content being learned to ask questions, plan investigations, make observations, make predictions, and offer explanations
-In my earliest science class experiences, we usually would develop some sort of experiment plan and a hypothesis. After learning about the content in class, we had the knowledge and skills to develop an experiment and solve problems. The scientific method is usually a corner stone of early science education.
Standard A 4.5: When studying a science-related problem, decide what changes over time are occurring or have occurred
-In high school biology, we performed a series of experiments on pea plants to see if water was the best way to get plants to grow. We would have to check our plant's growth progress every other day to see if changes have occurred. This allowed us to examine how the plants changed over time and how it was occurring.
B.4.2 Acquire information about people who have contributed to the development of major ideas in the sciences and learn about the cultures in which these people lived and worked.
-Throughout my k-12 science classes, we would learn about the scientists who discovered and developed the experiments/ theories that we were currently learning about. We would use various resources to help study these important scientists.
C.4.2 Use the science content being learned to ask questions, plan investigations, make observations, make predictions, and offer explanations
-In my earliest science class experiences, we usually would develop some sort of experiment plan and a hypothesis. After learning about the content in class, we had the knowledge and skills to develop an experiment and solve problems. The scientific method is usually a corner stone of early science education.
D.4.3. Understand that substances can exist in different states-solid, liquid, gas
- One of the first experiments I remember doing in middle school revolved around freezing and boiling water. This experiment helped my class understand that substances can exist in different forms of matter. This lead to us learning about different substances that can also do this.
E.4.1 Investigate that earth materials are composed of rocks and soils and correctly use the vocabulary for rocks, minerals, and soils during these investigations
-In 8th grade, our science classes revolved around the weather, space, and minerals/ the earth. We would learn about how rocks and minerals change over time and how they affect the planet. We also would learn about the correct vocabulary to be used with these.
F.4.3 Illustrate* the different ways that organisms grow through life stages and survive to produce new members of their type
-In various science classes we learned the basics about how animals reproduce and give birth. This included learning about how young species develop in their lifetime and various forms of birth (eggs, babies, etc.). Then in more advanced classes we learned about how plants reproduce and things like asexual reproduction.
G.4.3 Determine what science discoveries have led to changes in technologies that are being used in the workplace by someone employed locally
-In various science classes we went on field trips or had a quest speaker come in and talk to us about how technology has changed in their field. One that I remember fondly was going to the amusement park in Mall of America in order to learn more about physics and how the latest roller coaster technology worked.
H.4.1 Describe* how science and technology have helped, and in some cases hindered, progress in providing better food, more rapid information, quicker and safer transportation, and more effective health care
-In high school biology, we had to do research projects on how science have improved our day to day lives. I chose to do research on GMO's and how they could potentially solve world hunger if used correctly. I always enjoyed doing research on the latest scientific advancements.
New Standards Questions
1. Compared to previous standards, the new standards look like they will be more specific and complex than previous elementary science education. Students will start learning about energy and concepts like "Earth in the universe". I think the idea is that with the proper teaching/lesson plans, young students can learn more complex ideas than previously thought.
2. The concepts tend to have research and studying which can be applied to multiple disciplines (proper research skills are universal). The experiments dealing with energy will most likely use math and equations that students learn in other disciplines as well.
3. I think that the challenge for teachers teaching these new standards will be introducing the topics in ways that young students can understand. I believe that once students are properly introduced to these topics, they will be able to fully understand and learn them.
4. These standards are broad enough for teachers to get creative and make these experiments fun for students. If students are genuinely enjoying these topics, they will want to participate and learn actively. Some of the problems I had with my science classes were that my teachers didn't try and get students actively interested and participating. Instead students would just get through the experiments as fast as they could just to get the completion grade. If students are more engaged, they will be more invested in experiments.
Saturday, January 14, 2017
Assignment 3
Going left to right
1.Hydrogen Bromide (Bromane)
2. Dihydrogen Monoxide (Water, Oxidane)
3.Ammonia (Azane)



2. (Pictures Below)










1.Hydrogen Bromide (Bromane)
2. Dihydrogen Monoxide (Water, Oxidane)
3.Ammonia (Azane)


2. (Pictures Below)
aluminum carbide (Same name)
Al₄C₃
aluminum chloride (Same)
AlCl₃
aluminum hydroxide (Aluminium(3+) trioxidanide)
Al(OH)₃
ammonia (Azane)
NH₃
ammonium carbonate (Same)
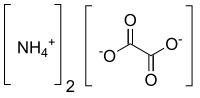
(NH₄)₂CO₃
ammonium cyanide (Same)
NH₄CN
ammonium hydroxide (azanium;hydroxide)
NH₄OH
ammonium nitrate (same)
NH₄NO₃
ammonium oxalate (Diammonium ethanedioate)
(NH₄)₂C₂O₄
ammonium phosphate (Same)
(NH₄)₃PO₄
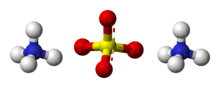
ammonium sulfate (Ammonium tetraoxosulfate)
(NH₄)₂SO₄
baking soda (Sodium hydrogen carbonate)
NaHCO₃
barium fluoride (Same)
BaF₂
barium hydroxide (Same)
Ba(OH)₂
barium phosphate (Same)
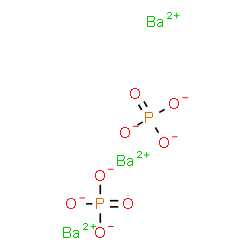
Ba₃(PO₄)₂
barium sulfide (Same)
BaS
beryllium chloride (same)
BeCl₂
beryllium nitrate (Same)
Be(NO₃)₂
boron trichloride (same)
BCl₃


















3. How many bonds does each of the following elements typically have? Carbon? Hydrogen? Oxygen?
Carbon: 4
Hydrogen: 1
Oxygen: 2
4.What does IUPAC stand for?
International Union of Pure and Applied Chemistry
International Union of Pure and Applied Chemistry
5.What chemicals are in the "chemical free" cleaner.
After performing research, I found out that the main ingredients for this cleaner are plant extracts, natural oils, and spearmint oil. The chemical make up for essential oils tend to be Terpene hydrocarbons and oxygenated compounds. The main chemicals in spearmint oil are as follows: The main components were carvone (51.7%) and cis-carveol (24.3%), followed by limonene (5.3%), 1,8 cineol (4.0%), cis-dihydrocarvone (2.2%), carvyl acetate (2.1%) and cis-sabinene hydrate (1.0%) (retrieved from Researchgate.net).
6. Chemical makeup of coffee.
After researching I discovered that there are over 1000 chemicals found in coffee. One of the more prominent ones found are chlorogenic acids.
Subscribe to:
Posts (Atom)









